 31
Jan,2026
31
Jan,2026
Why biosimilars matter more than you think
Imagine a life-saving drug that costs $20,000 a year. Now imagine the same treatment, just as safe and effective, costing $3,000. That’s not science fiction-it’s what biosimilars are doing right now in the U.S. healthcare system. Biosimilars aren’t generics. They’re not exact copies like the cheap pills you get for high blood pressure or cholesterol. Instead, they’re highly similar versions of complex biologic drugs-medicines made from living cells, used to treat cancer, autoimmune diseases, and other serious conditions. And they’re saving patients, employers, and the whole system billions.
As of January 2025, 10 FDA-approved biosimilars are available for Humira (adalimumab), the top-selling biologic in history. Some are priced up to 85% below the original list price. Yet, even with that kind of discount, originator biologics still control over 98% of spending. Why? Because the system isn’t built to reward savings-it’s built to protect them.
Biosimilars vs. generics: The big difference
When a brand-name pill like Lipitor loses its patent, a generic version can be made in a lab using simple chemistry. The active ingredient is identical. The pill looks the same. The cost drops 80-90% overnight.
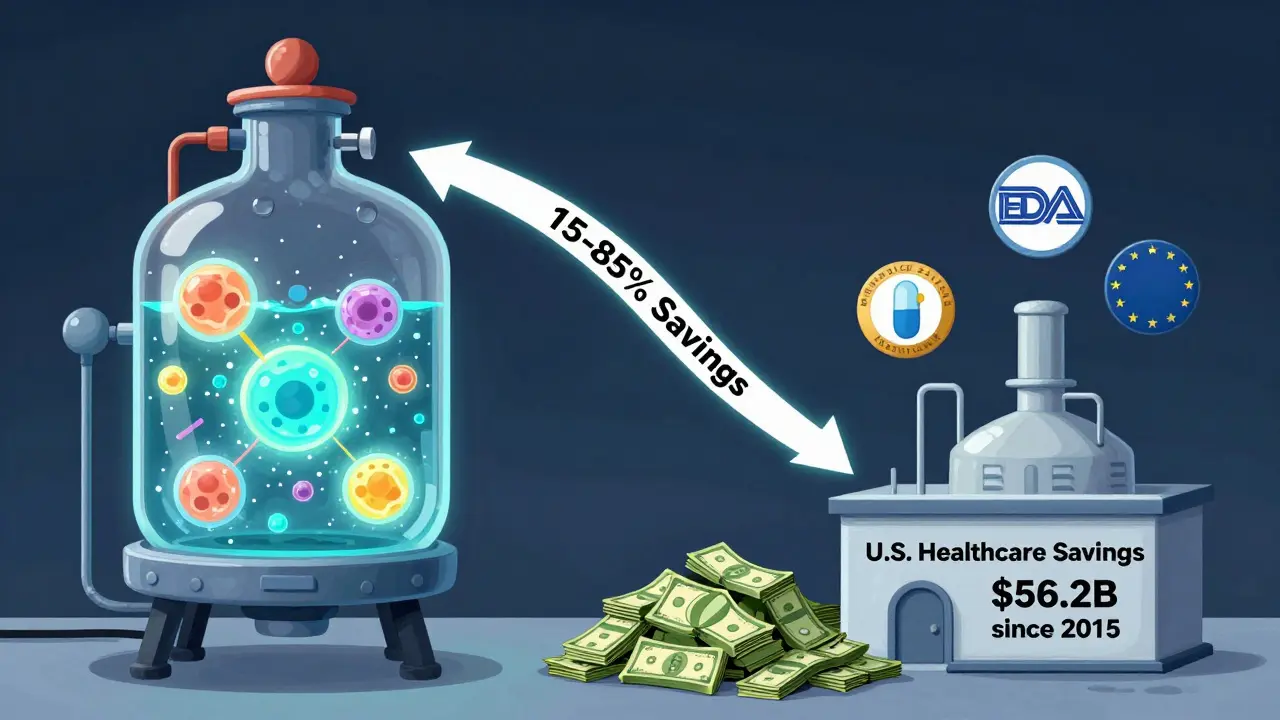
Biosimilars are different. They’re made from living cells-like yeast or hamster ovary cells-grown in giant bioreactors. Tiny changes in temperature, pH, or nutrients can alter the final product. That’s why biosimilars can’t be exact copies. They’re “highly similar,” with no clinically meaningful differences in safety or effectiveness. But because they’re so complex to make, development costs are much higher. That’s why their savings are usually 15-35% off the list price, not 80%.
That doesn’t mean they’re not powerful. It just means their impact works differently. Instead of one big price drop, biosimilars create steady pressure. As more enter the market, prices keep falling. The first Humira biosimilar cut prices by 30%. By 2025, with 10 options, some were selling for 85% less than the original list price.
Real savings, real numbers
The numbers don’t lie. In 2024 alone, biosimilars saved the U.S. healthcare system $20.2 billion. Since 2015, that total hits $56.2 billion. For perspective, that’s enough to cover the annual cost of insulin for every diabetic in Australia for nearly five years.
Take Humira again. Before biosimilars, it cost about $7,000 per month. Today, list prices for biosimilars range from $1,000 to $1,500. That’s an 85% drop. But here’s the catch: most patients don’t pay list price. Insurance and rebates muddy the waters. The actual net price-the amount insurers pay after rebates-is often much closer to the original. That’s why some patients still pay hundreds a month, even with a biosimilar.
But when you look at out-of-pocket costs, the picture changes. A 2025 study by CSRxP found that patients on biosimilars paid 23% less on average than those on the original biologic. For someone paying $500 a month for Humira, that’s $115 saved every month. That’s a car payment. Or groceries for a family for weeks.
Employers are seeing the same trend. One company saved $1.53 million in a single year just by switching all employees on Humira to a biosimilar. Across all self-insured U.S. employers, switching just two biologics to biosimilars could save $1.4 billion annually.
Why aren’t more people using them?
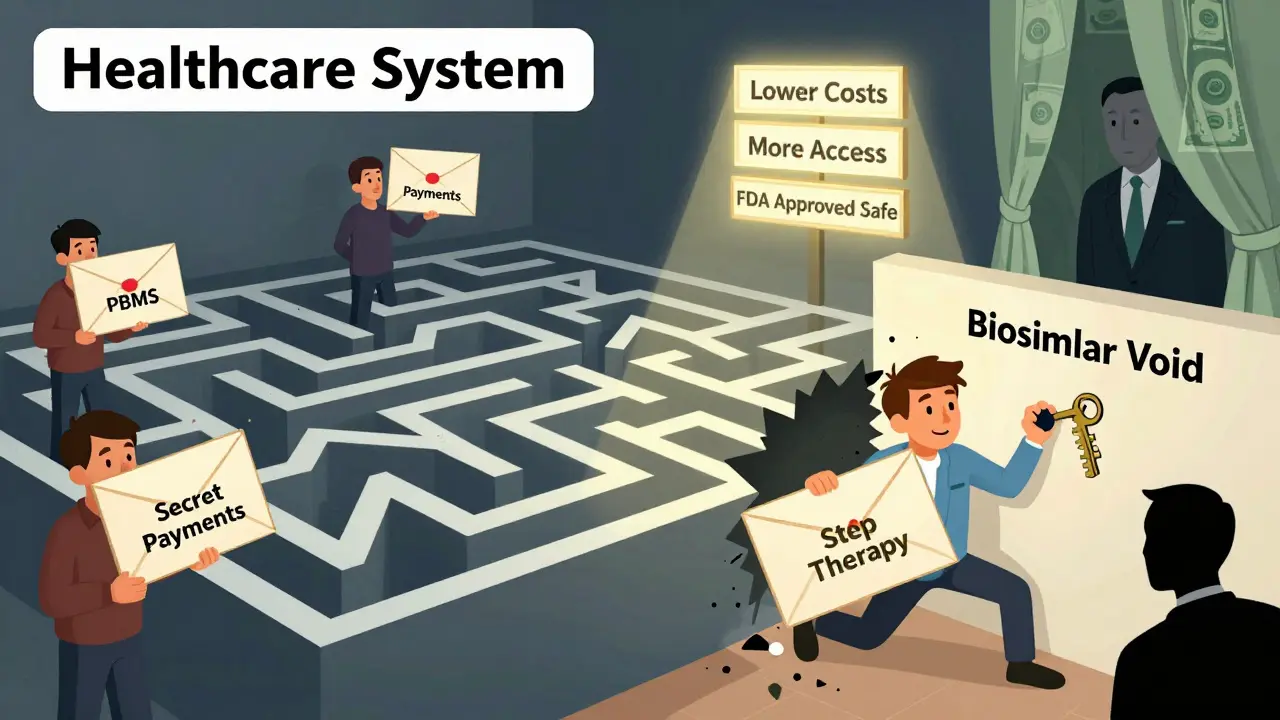
If biosimilars save money and work just as well, why do originator biologics still make up 98.9% of spending? The answer isn’t science-it’s money.
Big drugmakers use something called the “rebate trap.” They pay huge rebates to pharmacy benefit managers (PBMs) to keep their drug on the preferred list. Even if a biosimilar is cheaper, the rebate makes the originator look like the better deal on paper. PBMs, in turn, often push their own private-label biosimilars, which aren’t always the cheapest option.
Doctors and patients also have doubts. Some still think biosimilars are “second-rate.” But over 3.3 billion days of therapy have been given with biosimilars since 2015-with zero unique safety issues. The FDA and European regulators agree: they’re just as safe.
Then there’s the “biosimilar void.” Of the 118 biologics expected to lose patent protection over the next 10 years, only 12 have biosimilars in development. That means 90% of future savings are at risk. In Europe, 73% of high-sales biologics had biosimilars in the pipeline. In the U.S.? Just 23%.
How to get the most savings
If you’re an employer, insurer, or just someone paying for treatment, here’s how to unlock savings:
- Check your formulary. Ask your insurer or pharmacy benefit manager: Which biosimilars are covered? Are they preferred over the originator?
- Ask for step therapy. Many plans now require you to try a biosimilar first before approving the original. If yours doesn’t, ask your doctor to request it.
- Compare out-of-pocket costs. Don’t trust the list price. Ask your pharmacy for the final price after insurance and any coupons.
- Push for education. If your doctor is hesitant, ask for the latest clinical data. Studies in JAMA Network Open show biosimilar prices keep dropping over time after they enter the market.
For employers and health plans, the real win comes from contracts. Negotiate with PBMs to reduce rebates on originators and tie payments to biosimilar use. Use data tools like Segal’s SHAPE warehouse to track spending and spot where savings are being lost.
The bigger picture
Biologics are the most expensive drugs on the market. They treat cancer, rheumatoid arthritis, Crohn’s disease, psoriasis-conditions that can be life-altering. Without biosimilars, these drugs stay out of reach for millions.
But the U.S. is falling behind. Countries like Norway have over 80% biosimilar use for some drugs within three years. Here, adoption is slow. Why? Because the system rewards complexity over savings. Rebates, formulary games, and lack of policy pressure keep prices high.
There’s hope. The FDA has streamlined approval. The Inflation Reduction Act is pushing Medicare to negotiate prices. And in April 2025, the Biden administration made biosimilar competition a priority.
The math is simple: more biosimilars = lower prices = more patients treated. Right now, we’re leaving $234 billion in savings on the table over the next decade. That’s not a small gap. That’s a crisis in access.
Biosimilars aren’t perfect. But they’re the most powerful tool we have right now to bring down the cost of the most expensive medicines in modern medicine. The question isn’t whether they work. It’s whether we’ll let them.
Frequently Asked Questions
Are biosimilars as safe as the original biologics?
Yes. Every biosimilar approved by the FDA must show no clinically meaningful differences in safety, purity, or potency compared to the original. Over 3.3 billion days of patient use since 2015 have shown no unique safety concerns. Regulatory agencies in the U.S., Europe, and Australia all agree: biosimilars are just as safe.
Why don’t biosimilars cost 80% less like generics?
Because they’re not made the same way. Generics are simple chemical copies. Biosimilars are made from living cells, requiring complex manufacturing, strict quality controls, and years of testing. That makes development costlier. So while they don’t drop 80%, they still cut prices by 15-85%, depending on the drug and how many competitors enter the market.
Can I switch from a biologic to a biosimilar safely?
Yes, and many patients do. The FDA has approved some biosimilars as “interchangeable,” meaning pharmacists can substitute them without the doctor’s permission-just like generics. Even for non-interchangeable ones, doctors routinely switch patients with no loss of effectiveness. Studies show no increase in side effects or flare-ups when switching.
Why do some patients still pay a lot even with a biosimilar?
Because of rebates. Drugmakers pay big rebates to pharmacy benefit managers (PBMs) to keep their brand on preferred lists. Even if the biosimilar is cheaper, the rebate can make the original look like the better deal for the insurer. That means your out-of-pocket cost might not drop as much as expected. Always ask your pharmacy for the final price after insurance.
What’s the “biosimilar void” and why does it matter?
It’s the gap between biologics losing patent protection and biosimilars being developed to replace them. Of the 118 biologics expected to lose exclusivity in the next 10 years, only 12 have biosimilars in the pipeline. That means 90% of future savings are at risk. Without action, patients will keep paying high prices for drugs that could be much cheaper.
How can employers or health plans save more with biosimilars?
Start by reviewing your formulary. Push for step therapy that requires trying a biosimilar first. Negotiate contracts that reduce rebates on originators and reward biosimilar use. Use data tools to track spending. Educate doctors and patients. The savings are real-it’s just a matter of changing how the system works.



Been on a biosimilar for RA for two years now. Zero issues. My doc said it’s like swapping one brand of coffee for another-same caffeine, different bag. The savings? My copay dropped from $450 to $85. That’s a month of rent gone from my stress list.
Let’s be real: the system is rigged. PBMs get kickbacks, doctors get pressured, and patients? We’re stuck playing financial whack-a-mole. I had to fight my insurer for 3 months just to get switched to a biosimilar-despite the FDA saying they’re identical. Why does ‘savings’ only matter if it’s hidden in a rebate spreadsheet?
And don’t get me started on the ‘interchangeable’ label. It’s not a badge of honor-it’s a bureaucratic afterthought.
Wow. So the drug companies are evil, right? And the government’s gonna fix it? Please. They’re all in bed together. You think the FDA gives a damn? They approved 10 Humira biosimilars but didn’t touch the rebates. That’s not progress-that’s theater. If you want real savings, ban rebates. Not ‘educate doctors.’ Not ‘push step therapy.’ Just kill the kickbacks.
Just want to add: biosimilars aren’t magic, but they’re the best tool we’ve got. I work in a clinic and we’ve seen patients drop out of treatment because they can’t afford Humira. Since switching to biosimilars, adherence’s up 40%. The science is solid. The data’s there. It’s the biz model that’s broken.
Also, typo in the article: ‘hamster ovary cells’-yep, that’s real. No joke. Biotech is wild.
It’s interesting how we frame this as a ‘cost issue’ when it’s really about value systems. We assign worth based on origin, not outcome. A biosimilar isn’t ‘lesser’ because it’s not made by the same lab-it’s made by a different set of hands, with the same goal: to heal. Why do we equate price with purity? Is a painting less beautiful because it’s a replica?
Perhaps the real crisis isn’t the lack of biosimilars-it’s our refusal to see equality in difference.
Bro. Biosimilars = free money for patients. My cousin switched from Enbrel to a biosimilar and now he’s got cash for his kid’s braces. No side effects. No drama. Just cheaper. Why is this even a debate? We’re talking about people who can’t afford insulin. This isn’t politics-it’s survival.
Stop overcomplicating it. Just let people get the cheaper version. Done.
The numbers are screaming. $56 billion saved since 2015. $234 billion on the table over the next decade. We’re not talking about coffee. We’re talking about cancer treatments. Diabetes. Crohn’s. And we’re letting rebate games and PBM greed block access. This isn’t healthcare. It’s a casino where the house always wins. Time to burn the table.
Employers: stop letting PBMs dictate your benefits. Insurers: stop hiding behind ‘net price.’ Patients: demand transparency. And lawmakers: regulate the rebates. Or get out of the way.
Actually… biosimilars are a scam. 😏
Just kidding. Sort of.
But seriously-why are we still using the word ‘similar’? If it’s not identical, and we don’t know exactly how it behaves long-term… isn’t that just a fancy placebo with a lower price tag? 🤔
Also, who’s paying for the R&D on these? The same companies that made the originals? Hmmm…
How delightful. We’ve turned life-saving medication into a spreadsheet game. How quaint. How utterly American. I’m sure the shareholders are thrilled. Meanwhile, Mrs. Jenkins in Ohio is choosing between her Humira and her insulin. Let’s all take a moment to applaud the brilliance of our healthcare system. 🎉
Y’all are overthinking this. Biosimilars are cheaper. So what? The original still works better. I’ve seen patients flare after switching. And the ‘3.3 billion days’ stat? That’s just because they’re forcing it. No one’s tracking long-term immune response properly. It’s all marketing.
Also, why is everyone acting like this is new? It’s been 10 years. We’re still waiting for the ‘big drop.’
Actually, the FDA’s definition of ‘no clinically meaningful difference’ is incredibly rigorous. It’s not just ‘close enough.’ It’s statistical equivalence across multiple endpoints: immunogenicity, pharmacokinetics, efficacy, safety. The data is overwhelming. If you’re skeptical, read the actual clinical trial summaries. Not the blog posts.
And yes-rebates are the real villain. But blaming ‘Big Pharma’ without acknowledging the PBM monopoly is like blaming the chef for the restaurant’s broken AC.
Dear all, I have read this article with great interest. However, I must point out that the term 'biosimilar' is not officially recognized in the Indian regulatory framework. We use 'follow-on biologics' instead. Also, the data presented is U.S.-centric and may not be applicable globally. Furthermore, the phrase 'hamster ovary cells' is scientifically inaccurate-it should be 'CHO cells' (Chinese Hamster Ovary). Please be precise.
Just switched my husband to a biosimilar. Saved $1,200/month. 😍
I’m just glad this conversation is happening. My mom was on Humira for years. She never knew there was a cheaper option. I wish someone had told us sooner. Not everyone has the time or energy to dig through formularies. Maybe we need a simple tool-like a ‘biosimilar switch’ button on insurance apps?
i just want people to know they dont have to suffer because they cant afford it. i had a friend who stopped her meds because of cost. she got sicker. then she found a biosimilar. now she’s back to hiking. it’s not magic. it’s just fair.