21
Feb,2026
21
Feb,2026
FDA Serious Adverse Event Classifier
This tool helps you determine if a medical event would be classified as a serious adverse event (SAE) by the FDA based on their 5 specific criteria. Understanding this distinction is crucial for knowing when to report side effects and when they might not require immediate concern.
FDA Criteria for Serious Adverse Events
An event is classified as serious if any one of these 5 criteria is met:
- Death
- Life-threatening condition
- Hospitalization
- Disability or permanent damage
- Congenital anomaly or birth defect
Your event is not classified as a serious adverse event based on the FDA criteria.
When you start a new medication or join a clinical trial, you might see terms like "serious adverse event" or "SAE" in the paperwork. It sounds scary. But here’s the thing: not every bad reaction is a serious adverse event - and not every serious one feels severe. Understanding what the FDA means by "serious" can help you make smarter decisions, ask better questions, and know when to act - without panicking over side effects that aren’t actually dangerous in the way they’re defined.
What the FDA Really Means by "Serious Adverse Event"
The FDA doesn’t call every unpleasant side effect "serious." In fact, they have five very specific criteria that an event must meet to be classified as a serious adverse event (SAE). If any one of these happens, it’s considered serious - no matter how mild it might seem at first:
- Death - even if it’s only suspected to be linked to the drug or device.
- Life-threatening - meaning you were in real danger of dying at the time it happened. Not "could have been bad," but "this could have killed you right now."
- Hospitalization - either you had to go to the hospital because of it, or you stayed longer than planned. Just one extra night counts.
- Disability or permanent damage - something that messes up your normal life long-term. Think loss of vision, nerve damage, or inability to work or care for yourself.
- Congenital anomaly or birth defect - if you’re pregnant or planning to be, and the drug might affect a developing baby.
There’s also something called an "Important Medical Event" (IME). These are situations that don’t clearly fit the five criteria above - but doctors still think they could become serious if not treated. For example, a sudden drop in blood pressure that’s fixed with fluids, but could have led to organ failure. The FDA says: if a doctor says "this needs attention to prevent disaster," then it’s serious.
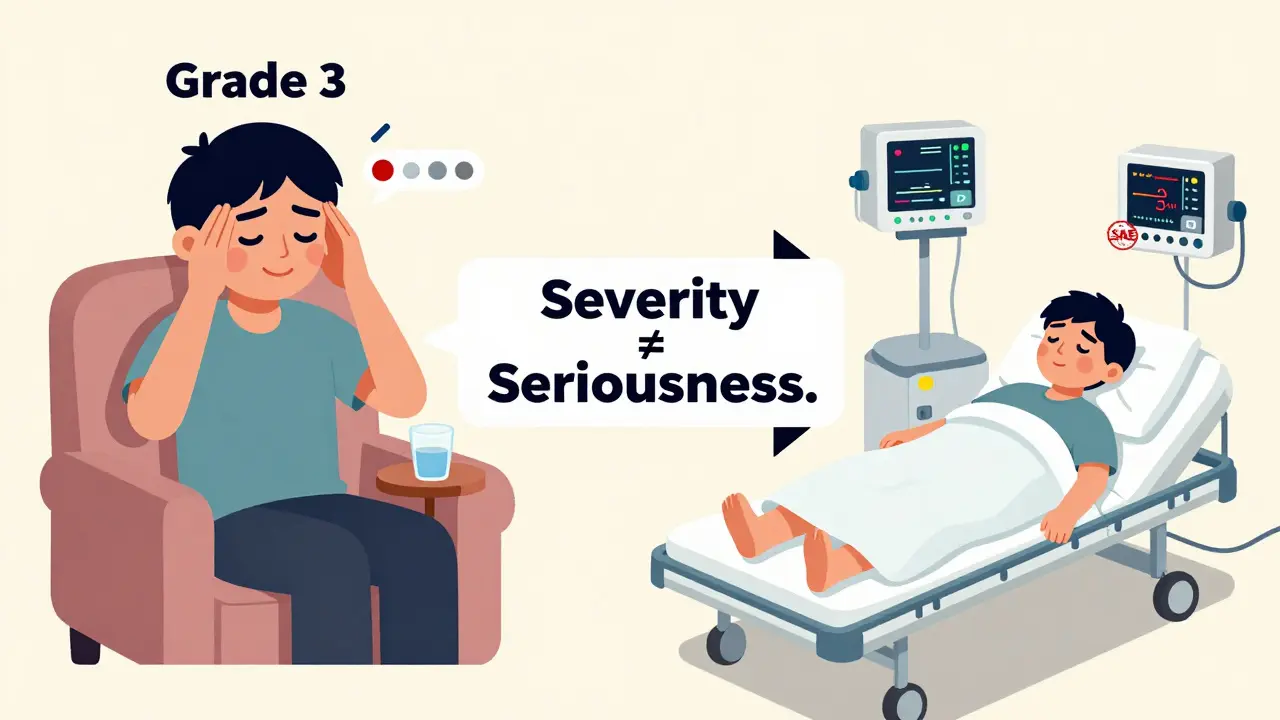
Why "Serious" Isn’t the Same as "Severe"
This is where most patients get confused. A lot of people think "serious" means "really bad," like a headache that feels like your skull is splitting. But the FDA doesn’t use "severity" to decide if something is serious. They use outcomes.
Here’s how they’re different:
- Severity is about how intense the symptom is - like mild nausea (Grade 1), moderate vomiting (Grade 2), or needing IV meds to stop it (Grade 3).
- Seriousness is about what the outcome was - did it land you in the hospital? Make you unable to walk? Threaten your life?
Example: You’re in a cancer trial. You get Grade 3 neutropenia - your white blood cell count crashes. It’s severe. But if your doctor gives you a quick shot of growth hormone and you’re back home in 48 hours? The FDA doesn’t call that serious. No hospitalization. No life-threatening risk. No permanent damage. So it’s not an SAE.
But if you had the same neutropenia, got a fever, and ended up in the ICU with sepsis? That’s a different story. That’s serious - even if the neutropenia itself was Grade 3.
A 2023 study by the American Society of Clinical Oncology found that 68% of severe side effects in cancer trials were not classified as serious. That’s because they didn’t trigger one of those five outcomes. Patients often panic when they see "Grade 3" on their report. But they shouldn’t - unless it led to hospitalization or worse.
How the FDA Uses This Info
The whole point of tracking serious adverse events isn’t to scare you. It’s to catch hidden dangers before they hurt more people.
Every time a drug manufacturer, doctor, or patient reports an SAE, it goes into the FDA’s Adverse Event Reporting System (FAERS). In 2022 alone, this system helped trigger 128 safety alerts and 47 changes to drug labels - like adding new warnings about heart rhythm problems or liver damage.
One big success? The FDA caught a pattern of rare but deadly liver injuries linked to a popular diabetes drug after five SAEs were reported over six months. The label was updated within 90 days. Patients were told to get liver tests before starting. That’s how this system saves lives.
And it’s getting smarter. The FDA’s Sentinel Initiative now watches health records from 300 million Americans. AI tools are being tested to flag patterns faster - cutting review time from 30 days to just 7 for the most urgent cases.
What You Can Do - As a Patient
You don’t need to be a scientist to understand this. Here’s what matters for you:
- Look for the five outcomes in any drug guide or consent form. If you see "hospitalization," "life-threatening," or "permanent damage," those are red flags - even if the side effect sounds minor.
- Ask: "Did this cause hospitalization or long-term harm?" Not "How bad did it feel?" That’s the real question the FDA asks.
- Report it yourself. The FDA’s MedWatch program lets patients report side effects directly. In 2022, over 38,000 reports came from patients - not doctors. Your report matters.
- Check for "Important Medical Events". If your doctor says "this isn’t serious, but we’re watching," trust them. It might not be an SAE, but it’s still important.
Many patients don’t realize they can report side effects on their own. You don’t need a doctor’s note. Just go to the FDA’s website, fill out Form 3500B, and describe what happened. It takes 10 minutes. And it helps improve safety for everyone.

What’s Changing - And What’s Coming
The FDA knows patients are confused. That’s why they’re making big changes.
- In 2023, they released draft guidance to use plain language in patient materials. Instead of "serious adverse event," they’ll say: "an event that caused death, hospitalization, disability, or life-threatening danger."
- By 2025, all clinical trial websites will have a simple summary of SAEs - no jargon.
- A new patient education portal is launching in late 2024. It’ll explain SAEs with real stories, videos, and checklists.
Also, patient voices are now shaping how SAEs are defined. In 2023, 78% of new drug approvals included input from patients about what they consider serious. For example, some patients said "chronic fatigue" - even if it didn’t hospitalize them - was life-altering. The FDA is now considering adding that as a new category.
Why This Matters for You
Understanding SAEs isn’t about memorizing government rules. It’s about knowing when to speak up.
If you’re on a new medication and you get dizzy - it’s probably not serious. But if you pass out and wake up in the ER? That’s an SAE. Report it. Tell your doctor. Ask if it’s been seen before.
And if you’re in a trial, don’t let scary terms like "Grade 4" scare you. Ask: "Did this cause hospitalization? Was I in danger of dying?" If the answer is no, it might not be serious - even if it felt awful.
Patients who understood this difference were 40% less likely to drop out of clinical trials, according to a 2023 survey. They weren’t afraid of side effects - they were informed.
The system isn’t perfect. Experts say only 1% to 10% of adverse events are reported. Some companies underreport. Some patients don’t know how. But every report you make - whether it’s your own or your parent’s - helps build a safer future for everyone taking medicine.
What’s the difference between a serious adverse event and a severe side effect?
A severe side effect describes how intense the symptom is - like nausea, pain, or fatigue. A serious adverse event is defined by the outcome: did it cause death, hospitalization, disability, life-threatening danger, or a birth defect? A side effect can be severe without being serious - like Grade 3 vomiting that’s treated at home. But even mild nausea can be serious if it leads to hospitalization from dehydration.
Do all bad reactions to medication get reported to the FDA?
No. Only serious adverse events (SAEs) must be reported by drug companies and clinical trial sites. Non-serious side effects - even common ones like headaches or dizziness - don’t have to be reported unless they’re part of a pattern. Patients can report any reaction through the FDA’s MedWatch program, but only SAEs trigger official safety reviews.
Can I report a side effect even if I’m not sure it’s serious?
Yes. The FDA encourages patients to report any unusual or concerning reaction, even if you’re unsure. If it turns out to be an Important Medical Event, it can still be flagged. Your report might help catch a pattern others missed. In 2022, 18,452 previously unreported events were identified because of patient reports.
Why do some serious side effects never show up on drug labels?
Some events are too rare to be detected in clinical trials, which usually involve a few thousand people. The FDA’s real-time monitoring system catches them later - through reports from doctors and patients after the drug is on the market. That’s why labels get updated years after approval. If a side effect affects fewer than 1 in 1,000 people, it might not appear until hundreds or thousands of reports come in.
How can I find out if a drug has had serious safety issues?
Check the drug’s FDA-approved prescribing information - usually found on the manufacturer’s website or the Drugs@FDA database. Look for the "Warnings and Precautions" section. It lists serious adverse events seen in trials. You can also search the FDA’s FAERS database for reports, though it’s technical. For plain-language summaries, wait for the new FDA patient portal launching in late 2024.
If you’re taking a new drug or joining a trial, keep this simple rule: don’t judge danger by how bad it feels. Judge it by what it did to you. Hospitalized? Life-threatening? Permanent? Then it’s serious. And you should report it.