 26
Jan,2026
26
Jan,2026
Many people see a prescription for a generic drug and wonder: Is this really the same as the brand-name medicine they’ve always taken? The answer is yes - but only if you understand what that really means. Too often, patients are handed a small white pill with no name on it and told, "It’s the same thing." That’s not enough. Without clear, simple explanations, people stop taking their meds, switch back to expensive brand names, or skip doses altogether. This isn’t just about money - it’s about trust, safety, and getting better.
What exactly is a generic drug?
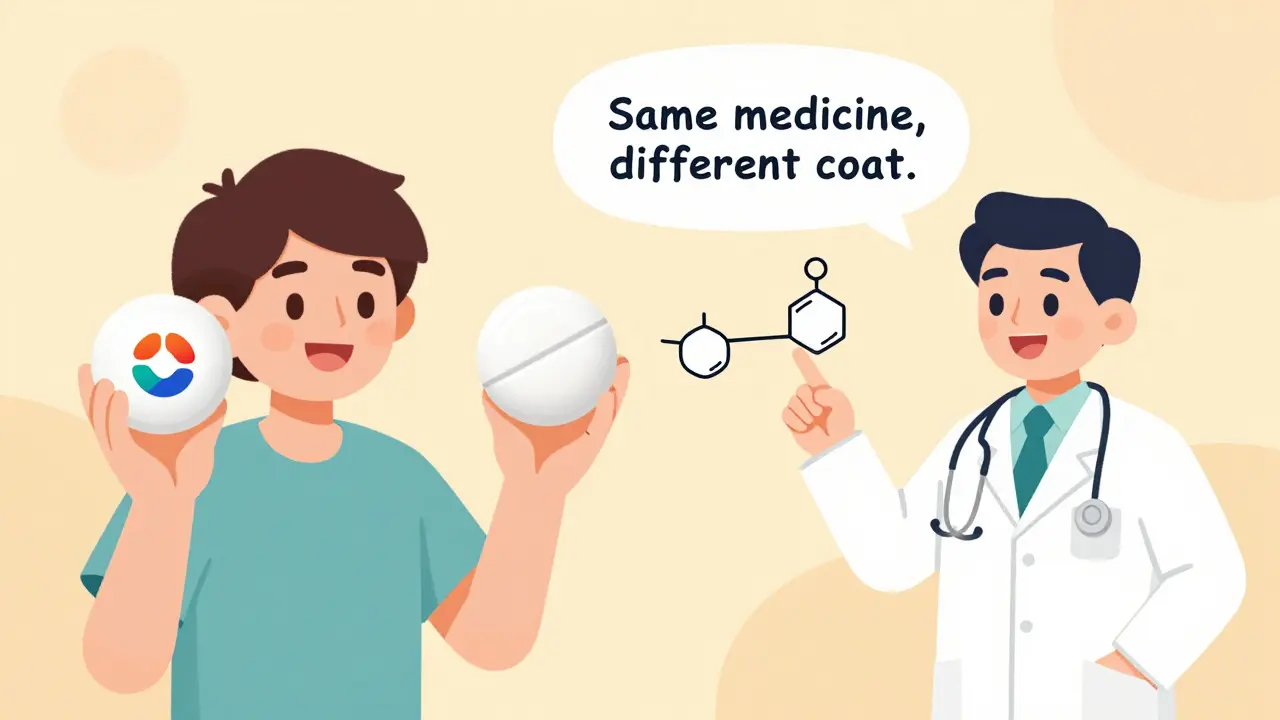
A generic drug is not a copy. It’s not a knockoff. It’s the exact same medicine, made to meet the same strict standards as the brand-name version. The FDA requires that generic drugs have the same active ingredient, strength, dosage form, and route of administration. That means if your brand-name pill is 500mg of amoxicillin taken by mouth, the generic is also 500mg of amoxicillin taken by mouth. No exceptions.The key difference? The inactive ingredients - things like dyes, fillers, or coatings. These don’t affect how the drug works. They might change the color or shape of the pill, or make it easier to swallow. But the part that treats your infection, lowers your blood pressure, or controls your thyroid? That’s identical.
Here’s a simple way to think about it: Tylenol is to acetaminophen what Kleenex is to tissues. One is a brand name. The other is the actual product inside. You’re getting the same thing - just without the marketing.
How do we know generics work the same?
The FDA doesn’t just approve generics based on paperwork. They require proof - real, measurable proof. Before a generic drug hits the shelf, it must show it delivers between 80% and 125% of the same amount of medicine into your bloodstream as the brand-name version. That’s called bioequivalence. It’s not a guess. It’s tested in healthy volunteers using blood samples and precise lab equipment.Between 2010 and 2020, out of 11,000 generic drugs approved by the FDA, 98.7% met this standard. That’s not close - that’s nearly perfect. And since 2009, generic drugs have saved the U.S. healthcare system nearly $2 trillion. If they didn’t work, those savings wouldn’t exist.
Some people worry about switching from brand to generic. They’ve heard stories of feeling different. But for most drugs - like statins, antibiotics, or blood pressure pills - studies show no difference in outcomes. A 2016 study of over 8,600 patients taking generic atorvastatin (the generic version of Lipitor) found the same results in lowering cholesterol and preventing heart attacks as the brand name.
When generics might need extra care
Not every drug is the same across brands - even if they’re both generic. Some medications have what’s called a narrow therapeutic index. That means the difference between the right dose and a harmful one is very small. For these, even tiny changes in how the body absorbs the drug can matter.Drugs like warfarin (a blood thinner), levothyroxine (for thyroid problems), and phenytoin (for seizures) fall into this category. In these cases, doctors may recommend sticking with one brand or generic version - not because generics are unsafe, but because consistency matters. If you switch between different generic versions of levothyroxine, your thyroid levels might fluctuate. That’s why the American Association of Clinical Endocrinologists advises patients on this drug to use the same manufacturer whenever possible.
And yes, there have been exceptions. In 2012, the FDA pulled one generic version of Wellbutrin XL after finding it didn’t release the drug properly. That’s why the FDA still monitors generics after they’re on the market. But these cases are rare - less than 0.1% of all generics ever approved.
Why do people still distrust generics?
Despite the science, 38% of Medicare beneficiaries still believe generics are less effective. Why? Because they’ve been told they are. Or they’ve seen the pill look different. Or they’ve heard a rumor from a friend. Or they assume cheaper means worse.Pharmacies and health systems are fighting this with simple tools called consumer language guides. These aren’t dense FDA documents. They’re short, visual, plain-language handouts. One popular tool from the FDA shows side-by-side images of brand and generic pills with labels like:
- Brand: Nexium - active ingredient: esomeprazole
- Generic: esomeprazole - same active ingredient
These guides use analogies, pictures, and clear headings. They avoid words like “bioequivalence” and “pharmacokinetics.” Instead, they say: “This medicine works the same way. It’s just cheaper.”
A 2021 study found patients who got these guides understood the difference 37% better than those who got standard medication sheets. And when pharmacists spend just 90 seconds explaining generics using three simple points - same active ingredient, same effect, big cost savings - patients are more likely to stick with the generic.
What’s the real cost of not understanding?
When people don’t trust generics, they pay more. And that’s not just their wallet. It’s the whole system. In 2023, the Association for Accessible Medicines estimated that confusion over generics led to $3.2 billion in unnecessary brand-name prescriptions each year.Some patients switch back to brand-name drugs after a few weeks because they feel “something’s off.” But often, it’s not the drug - it’s the pill’s shape, color, or size. A patient might think, “This isn’t the one my doctor gave me,” and stop taking it. That’s dangerous. For someone with high blood pressure or diabetes, skipping doses can lead to hospital visits - which cost far more than the difference between brand and generic.
On the flip side, when patients understand generics, they take their meds more consistently. The FDA found that when patients get a clear explanation, adherence goes up by 22%, and switch-back rates drop by 34%.

What should you do?
If you’re prescribed a generic drug:- Ask your pharmacist: “Is this the same as the brand name?” They’ll show you the comparison chart.
- Check the pill’s shape and color - but don’t assume a change means it’s different. Ask if the manufacturer changed.
- Don’t stop taking it because it looks different. Call your pharmacy or doctor first.
- For high-risk drugs like thyroid medicine or seizure meds, ask if you should stick with one brand or generic.
- Use the FDA’s website - they have free, updated guides with real examples.
And if you’re a caregiver, parent, or someone helping an older adult - don’t assume they understand. Show them the guide. Explain it in plain words. Use the cereal analogy. Ask them to repeat back what they heard. That’s called the “teach-back” method - and it works.
What’s next for generic drug education?
The FDA just launched a new initiative to create personalized guides for high-risk drugs like levothyroxine and warfarin. Starting in 2025, Medicare Part D plans will be required to give all patients standardized education materials that meet health literacy standards. Pharmacies are training staff to use the same three-point explanation every time. And AI tools are being tested to tailor messages based on how well someone reads or understands medical terms.The goal isn’t just to sell more generics. It’s to make sure people get the medicine they need - without fear, confusion, or unnecessary cost. Because when patients understand what they’re taking, they take it. And when they take it, they stay healthy.
Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires generic drugs to meet the same safety and quality standards as brand-name drugs. They’re made in the same type of facilities, tested the same way, and monitored after they’re on the market. The only difference is the price - not the safety.
Why do generic pills look different?
Generic pills look different because U.S. law says they can’t look exactly like the brand-name version. That means different colors, shapes, or markings. But the active ingredient - the part that treats your condition - is identical. The differences are only in fillers, dyes, or coatings, which don’t affect how the drug works.
Can I switch between different generic brands?
For most drugs, yes. But for medications with a narrow therapeutic index - like warfarin, levothyroxine, or phenytoin - it’s best to stick with one manufacturer. Small changes in how the body absorbs the drug can affect your treatment. Talk to your doctor or pharmacist if you’re unsure.
What’s an authorized generic?
An authorized generic is the exact same drug as the brand name, just sold without the brand label. It’s made by the same company that makes the brand version. These often have the lowest switch-back rates because they’re identical in every way - including how they look and feel.
How much money can I save with generics?
On average, you’ll save about $387 per prescription compared to the brand-name version. For chronic conditions like high blood pressure or diabetes, that adds up to thousands of dollars a year. In 2022, generics made up 90.9% of all prescriptions filled in the U.S. but only 22.3% of total drug spending.
Where can I find reliable information about my generic drug?
The FDA’s website has free, updated guides called "Generic Drug Facts." You can also ask your pharmacist for a consumer language guide - most major pharmacies now provide them. Avoid relying on social media or random websites. Stick to trusted sources like the FDA, CDC, or your pharmacy.
What to do if you’re still unsure
If you’re still nervous about your generic prescription, don’t guess. Call your pharmacy. Ask them to show you the comparison chart. Ask if your drug is one of the rare ones that needs extra care. Ask if an authorized generic is available. You have the right to understand what you’re taking - and you don’t need a medical degree to get that answer.The system is designed to save you money - but only if you know how to use it. Don’t let confusion cost you your health.

I used to freak out when my pill changed color. Then I started asking my pharmacist to show me the comparison sheet. Turns out, it’s the same stuff. Just cheaper. I saved like $400 this year on my blood pressure med. No side effects, no weird feelings. Just… less money out of my pocket. 🙌
My grandma switched to generic insulin last year and now she’s got extra cash for bingo night. She still calls it ‘the white pill’ and swears it’s magic. Honestly? It is.
Let’s deconstruct the epistemological framework of pharmaceutical equivalence, shall we? The FDA’s bioequivalence standard of 80–125% AUC is not a validation of therapeutic identity-it’s a statistical loophole masquerading as scientific consensus. The active ingredient may be identical, but the excipient matrix-those ‘inert’ fillers-is a pharmacokinetic wildcard. When you factor in individual microbiome variance, CYP450 polymorphisms, and the placebo/nocebo effect as a biological variable, the assertion that ‘it’s the same’ collapses under its own ontological weight. We’re not talking about cereal-we’re talking about the delicate dance of molecular bioavailability in a human system that doesn’t care about your cost-saving agenda.
My mom took her thyroid med for 15 years on the same brand then switched to generic and never noticed a difference. She says the pill looks weird but it still works. Just ask your pharmacist. Easy.
Oh wow so the FDA says it’s fine? How convenient. You know what else the FDA said was fine? Vioxx. And thalidomide. And those vape pens that blew up in people’s pockets. They’re not protecting you-they’re protecting Big Pharma’s bottom line. Generic manufacturers are the ones cutting corners. The 2012 Wellbutrin recall? That was just the tip of the iceberg. You think they test every batch? Nah. They test one batch from India and call it a day. Your ‘same medicine’ is probably laced with talc and wishful thinking.
AMERICA MADE THE BRAND. AMERICA DESERVES THE BRAND. WHY ARE WE LETTING FOREIGN FACTORIES MAKE OUR MEDS?? 🇺🇸💊 #BuyAmerican #GenericIsGimmick
If you’re too lazy to pay for the real thing, don’t blame the system when you feel weird. People who take generics are the same people who buy store-brand toilet paper and wonder why it falls apart. You get what you pay for. Period.
Anna Lou Chen’s comment above is technically correct but misses the point entirely. Bioequivalence isn’t about philosophical purity-it’s about clinical outcomes. The 2016 atorvastatin study with 8,600 patients showed no difference in LDL reduction or cardiovascular events. That’s not statistics. That’s real people. If your thyroid levels fluctuate after switching generics, that’s a monitoring issue, not a systemic failure. The FDA’s post-market surveillance catches 99.9% of issues before they become problems. Your fear is not data.
It’s wild how something so simple-like knowing your pill works the same-can save lives. People are scared because they don’t understand. Not because it’s dangerous. Just because no one took the time to explain it. I’ve handed out those FDA handouts to three friends this month. All of them switched and are saving hundreds. We just need to talk about it more.
Let’s be real-generics are the unsung heroes of modern medicine. You don’t hear about them because they’re not flashy. No celebrity ads. No shiny packaging. Just a little white pill doing its job while you save your rent money. I used to think the color change meant something was off… until I learned that the dye in the brand-name version was actually a carcinogen in high doses. The generic? No dye. Just pure medicine. Sometimes cheaper means better. Not just for your wallet-for your body too.
Interesting how the post frames the issue as one of education, yet ignores the structural coercion at play. Pharmacies are incentivized to substitute generics regardless of patient preference. Insurance formularies penalize prescribers who don’t choose the cheapest option. This isn’t empowerment-it’s economic triangulation disguised as patient advocacy. The real question isn’t whether generics work-it’s whether patients ever had a real choice to begin with.