 21
Dec,2025
21
Dec,2025
Carbamazepine is one of those medications that works well for many people - until it doesn’t. Whether you’re taking it for epilepsy, nerve pain, or bipolar disorder, the real challenge isn’t just getting the right dose. It’s staying on the same version. Generic carbamazepine might look and cost the same, but under the surface, small differences can trigger big problems. And the reason? Enzyme induction and a narrow therapeutic window.
Why carbamazepine is tricky from the start
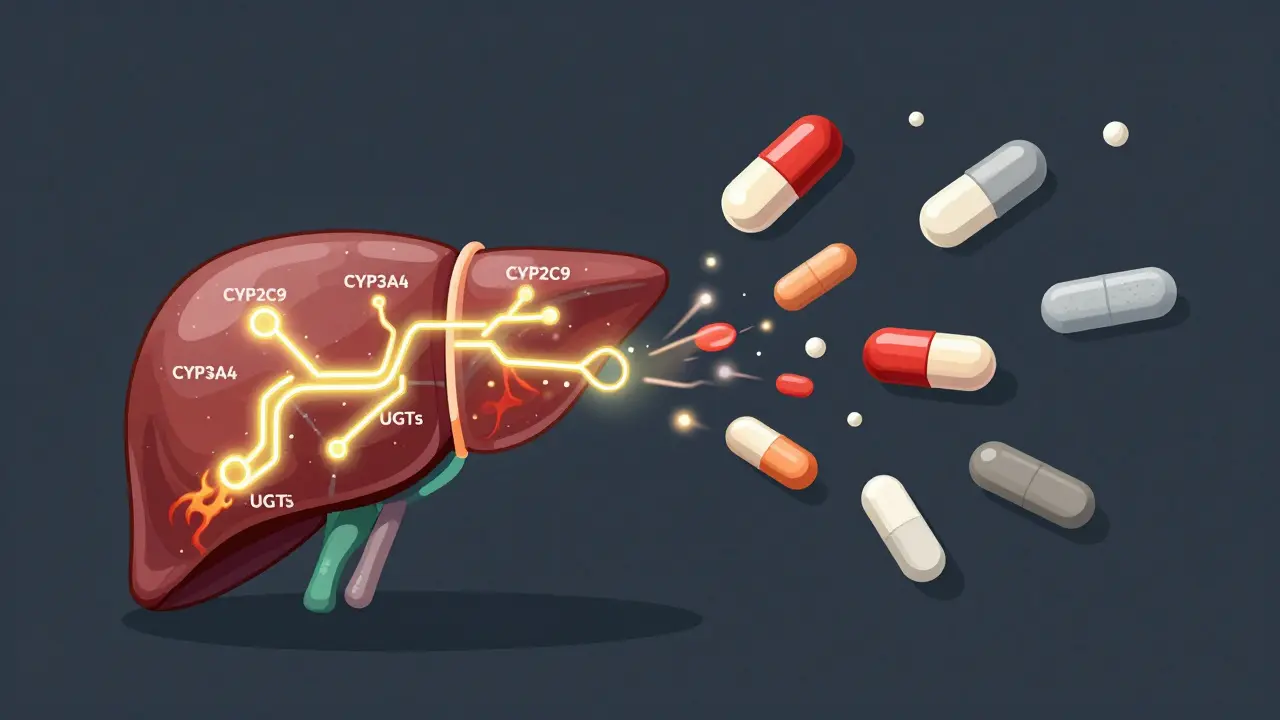
Carbamazepine doesn’t just sit in your body. It changes how your body works. It turns on enzymes - specifically CYP3A4, CYP2C9, and UGTs - that break down not just carbamazepine itself, but also other drugs you might be taking. This is called autoinduction. Within 48 to 72 hours of starting the drug, your liver starts working harder to clear it. By two to three weeks, you’re metabolizing it up to 50% faster than when you first began.This means the dose you started with may stop working. Your blood level drops. Seizures return. Pain flares up. Mood swings creep back in. That’s why therapeutic drug monitoring (TDM) is standard practice for carbamazepine. About 7 out of 10 people on this drug need regular blood tests to make sure levels stay between 4 and 12 mcg/mL. Go below 4, and the drug stops being effective. Go above 12, and you risk dizziness, nausea, blurred vision, or worse - toxic reactions.
Generic versions aren’t all the same - even if they’re approved
All generic carbamazepine must prove they’re bioequivalent to the brand-name version (Tegretol). That means in healthy volunteers, their absorption (AUC) and peak concentration (Cmax) must fall within 80-125% of the original. Sounds fair, right?Here’s the catch: those studies are done on healthy people. Not on someone with epilepsy, liver disease, or who’s taking five other meds. And they’re done after one dose. Carbamazepine’s autoinduction doesn’t show up until weeks later.
A 2018 study tracked 327 patients who switched between different generic brands. Over 12% had seizures or side effects serious enough to visit the ER. Why? Because even small differences in how the tablet dissolves - the size of the beads in extended-release capsules, the coating, the filler - can change how fast the drug enters your system. For a drug with a narrow window like carbamazepine, that’s enough to tip the balance.
One patient on Reddit, ‘NeuroNurse2020,’ noticed her extended-release capsules from Nostrum Pharmaceuticals had different bead sizes than other generics. That mattered because she had gastroparesis - slow stomach emptying. The beads didn’t dissolve properly, so her levels dropped. She didn’t know why her seizures got worse - until she checked the label.
Who’s most at risk when switching generics?
Not everyone has problems. About 60% of people switch generics without issue. But certain groups are far more vulnerable:- Women of childbearing age: Hormones affect CYP3A4 activity. A 2021 JAMA Neurology study found women had 22% more breakthrough seizures after switching generics than men.
- People on multiple seizure drugs: When carbamazepine is mixed with valproate, lamotrigine, or topiramate, the variability in blood levels jumps from 25% to 45%. Small formulation differences become dangerous.
- Patients with prior instability: If you’ve ever had a seizure after a dose change, or if your seizures are still not fully controlled, switching generics is risky.
- People of Asian descent: The FDA warns that those with the HLA-B*1502 gene variant have a 10 times higher risk of Stevens-Johnson Syndrome - a life-threatening skin reaction - when taking carbamazepine. Screening is recommended before starting, especially for those from Southeast Asia.
Doctors who treat epilepsy know this. The American Academy of Neurology’s 2019 guidelines say: don’t switch carbamazepine in patients with poorly controlled seizures. Yet pharmacies still substitute automatically unless the doctor writes "dispense as written" (DAW 1) on the prescription. Only 68% of U.S. neurologists do this consistently.
What happens when you switch - and how to catch it early
If you’re switched from one generic to another, don’t wait for a seizure to happen. Watch for these signs:- New or worsening dizziness, blurred vision, or unsteadiness
- Increased seizure frequency or intensity
- Unexplained rash, fever, or sore throat (possible early sign of blood toxicity)
- Mood changes, confusion, or memory lapses
And get your blood tested. The International League Against Epilepsy recommends checking carbamazepine levels:
- Before the switch (baseline)
- 7 to 10 days after the switch
- 4 weeks after the switch
If your level changes by more than 15%, your dose needs adjusting - even if you feel fine. A drop from 7.2 to 4.8 mcg/mL, like one patient reported, means you’re no longer protected. Your doctor might need to increase the dose, or switch you back to the original brand.
How to protect yourself
You don’t have to accept random switches. Here’s what you can do:- Ask your doctor to write "DAW 1" on your prescription. This tells the pharmacy to dispense the exact brand or generic you’re on - no substitutions.
- Check the label every time you refill. Look for the manufacturer name. If it changes, call your doctor before taking it.
- Keep a seizure or symptom diary. Note when you switch, what dose you’re on, and any changes in symptoms. This helps your doctor spot patterns.
- Know your HLA-B*1502 status. If you’re of Asian descent, ask if you’ve been tested. If not, get it done before starting or switching.
- Don’t assume generics are interchangeable. Even if two are both labeled "carbamazepine 200 mg," they may not behave the same in your body.

The future: precision dosing and better science
Regulators are starting to catch up. The FDA now calls carbamazepine extended-release products "high-priority" for better testing. New guidelines require dissolution tests across multiple pH levels and demand steady-state bioequivalence studies - not just single-dose ones.Research is also moving toward personalized dosing. Scientists have found 17 gene variants that affect how fast people metabolize carbamazepine. People with the CYP3A4*22 variant need 25% less drug to reach the same level. Future tools will combine genetic data, weight, age, sex, and other meds into algorithms that predict the right dose before you even start.
For now, though, the safest approach is simple: stay on the same version. If your doctor says switching is okay, ask for a blood test before and after. If your pharmacy changes your pill without telling you, speak up. Your health isn’t a cost-saving experiment.
What to do if you’ve already switched
If you’ve recently changed from one generic carbamazepine to another and feel off:- Don’t stop the drug suddenly - that can trigger seizures.
- Call your neurologist or epilepsy specialist. Tell them you switched generics.
- Request a serum level test within 7 days.
- If levels dropped or side effects started, ask to go back to your previous brand or generic.
Many people get better once they return to the formulation they were on. But you have to catch it fast.
Can I switch between different generic carbamazepine brands safely?
It’s possible, but not always safe. About 1 in 8 people experience problems like breakthrough seizures or side effects after switching. The risk is higher if you have epilepsy that’s not fully controlled, are a woman of childbearing age, or take other medications. Always check your blood levels before and after switching, and only change if your doctor approves and monitors you.
Why does carbamazepine interact with so many drugs?
Carbamazepine strongly activates liver enzymes - especially CYP3A4 - that break down many common medications. This includes blood thinners like warfarin, cholesterol drugs like atorvastatin, birth control pills, antidepressants, and even some antibiotics. As a result, those drugs become less effective. If you’re on carbamazepine, always tell your doctor about every other medication, supplement, or herb you take.
How long does it take for carbamazepine to start affecting other drugs?
Enzyme induction begins within 2 to 3 days, but it takes 2 to 3 weeks to reach full strength. That’s why interactions often show up weeks after starting or changing carbamazepine - not right away. Even after stopping the drug, enzyme activity stays elevated for 1 to 2 weeks, so interactions can continue during that time.
Is there a blood test to check if carbamazepine is working?
Yes. A serum carbamazepine level test measures how much drug is in your blood. The goal is 4-12 mcg/mL. Levels below 4 may mean the drug isn’t working. Levels above 12 increase the risk of side effects. This test is especially important after starting, changing doses, or switching between generic brands.
Should I avoid carbamazepine if I’m Asian?
Not necessarily, but you should be screened first. If you have the HLA-B*1502 gene variant - common in people of Chinese, Thai, Malaysian, or Filipino descent - your risk of a severe skin reaction (Stevens-Johnson Syndrome) is 10 times higher. If you test positive, your doctor will likely choose a different medication. If you test negative, carbamazepine can still be used safely.
Can I take birth control while on carbamazepine?
Carbamazepine reduces the effectiveness of hormonal birth control by up to 50%. You should use a non-hormonal method - like an IUD or condoms - as your primary form of contraception. If you must use hormonal birth control, your doctor may need to increase the dose, but even then, effectiveness isn’t guaranteed. Always talk to your doctor about your options.



Just switched generics last month and my seizures got worse. Didn’t think it was the med until I checked the label. Now I’m back on my old brand and life’s back to normal.
They say bioequivalent but the bead size difference in extended-release? That’s a joke. My cousin in Bangalore had a seizure because the filler changed. Pharma companies don’t care as long as the label says 'carbamazepine'.
India makes the cheapest generics. You think they test this stuff? My uncle took a batch from a factory that used recycled plastic in the coating. He ended up in the ICU. This isn’t medicine - it’s lottery.
Carbamazepine’s autoinduction is wild. It’s like your liver learns to hate you after a few weeks. And switching generics? It’s like changing your car’s fuel mid-roadtrip and hoping the engine doesn’t blow. The science is solid - the system isn’t.
Doctors know this. Pharmacies don’t care. Patients pay the price. We need mandatory pre/post TDM for any switch. Not optional. Mandatory.
I’ve been on carbamazepine for 12 years. Switched generics three times. Each time, I felt like I was slowly losing myself - dizziness, brain fog, mood swings. I kept thinking it was stress, or aging, or depression. Turns out, it was the damn beads in the capsule.
One brand had larger beads, slower dissolution. Another used a coating that dissolved too fast in my acidic stomach. My levels swung from 3.8 to 11.4 in three weeks. I didn’t know what was happening until I read this post. Now I take a picture of the label every refill. I don’t trust anything anymore.
It’s alarming how little oversight exists for generic bioequivalence in chronic, narrow-therapeutic-index drugs. The FDA’s current standards were designed for acute conditions, not lifelong neuropharmacology. Single-dose studies in healthy volunteers are statistically inadequate for predicting real-world outcomes in polypharmacy, comorbid, or metabolically variable populations. This is a systemic failure, not a manufacturing error.
Big thanks for this. I’ve been telling my neurologist for years that generics aren’t interchangeable. She said ‘they’re all the same.’ I showed her this. Now she writes DAW 1 on everything. My levels are stable again. You’re not crazy if you feel off after a switch - you’re just paying attention.
Man, this is why we can't trust foreign meds. In Nigeria, we get generics from everywhere - India, China, even Turkey. One batch gave me rashes so bad I couldn’t sleep. My doctor said ‘it’s just allergy.’ I knew better. I switched back to the old one. No more rashes. No more seizures. Same pill, different maker. That’s the problem.
U.S. generics are a scam. We pay more for brand-name drugs than other countries, then get slapped with random generics that don’t work. It’s corporate greed. I’m done with this system. I’m moving to Canada. At least there, they test the damn pills properly. 🇨🇦✊
It is imperative to underscore that the regulatory framework governing bioequivalence for narrow therapeutic index medications remains fundamentally inadequate. The 80-125% confidence interval, derived from healthy adult populations, is statistically inappropriate for clinical populations exhibiting pharmacokinetic variability due to polypharmacy, hepatic impairment, or genetic polymorphisms. This represents a critical gap in pharmacovigilance protocol.
I’m a nurse in Texas. I’ve seen this too many times. Patient comes in with new seizures. We check meds - switched generics last week. Blood level’s half of what it was. We switch back. Done. It’s not rocket science. Why do we make patients suffer because of pharmacy automation?
carbamazepine is so finicky. i switched to a new generic and thought i was just tired. turns out my level was 3.1. i almost had a seizure at work. now i check the label like it’s a lottery ticket. also, birth control? forget it. i got an iud and never looked back.
Here’s the uncomfortable truth: we treat drugs like commodities. But carbamazepine isn’t aspirin. It’s a scalpel. And we’re letting pharmacists hand out different blades every week and calling it ‘choice.’ This isn’t capitalism - it’s medical negligence wrapped in a cost-cutting slogan. If your life depends on a stable blood level, you don’t get to choose the brand. You deserve consistency. Period.