 27
Dec,2025
27
Dec,2025
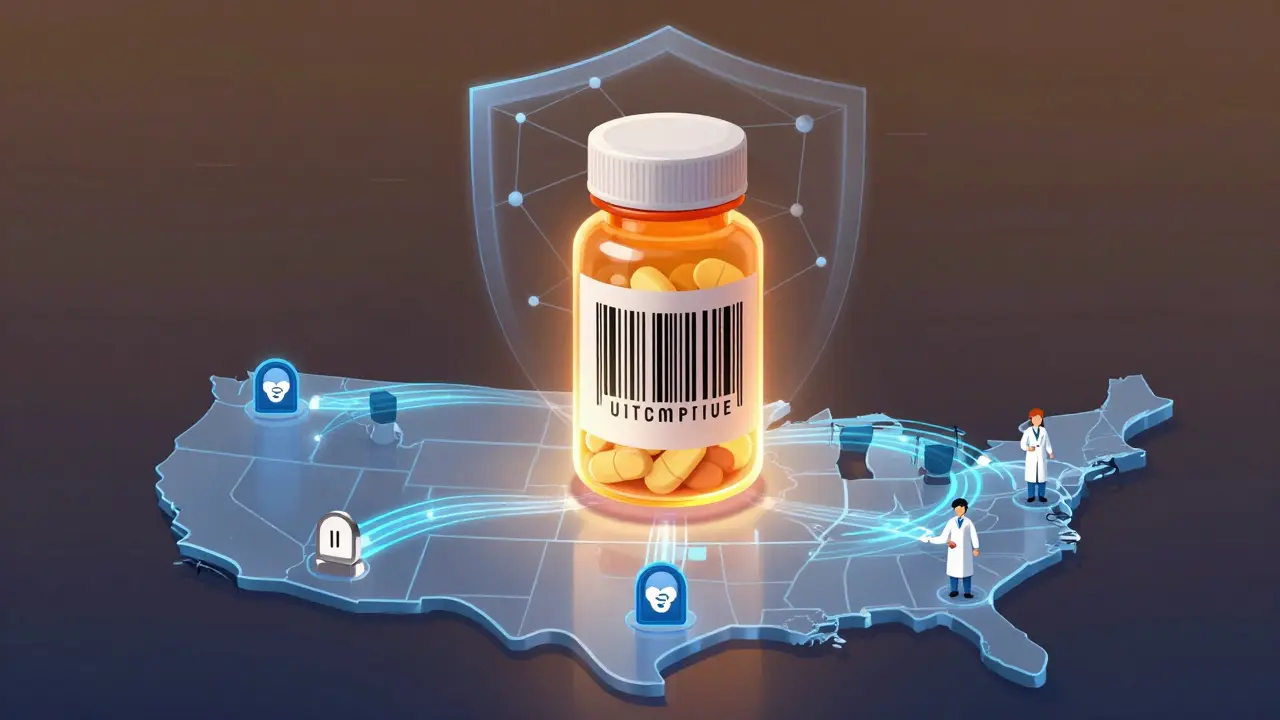
Every year, more than 5.8 billion prescription drug packages move through the U.S. supply chain-from factories to pharmacies, then into the hands of patients. But how do we know those pills, injections, and capsules are real? Not fake. Not contaminated. Not stolen? The answer isn’t just about police raids or warning labels. It’s a quiet, high-tech system built over a decade to stop counterfeit drugs before they ever reach you.
What’s at stake when drugs go fake
Counterfeit drugs aren’t just illegal-they’re deadly. They might contain no active ingredient, too much of it, or toxic chemicals like rat poison or paint thinner. The FDA has seized over 1,100 fake drug packages in 2014. By 2022, that number dropped to 412. That’s a 63% decline. Not because people stopped making fakes. But because the system to catch them got smarter. The problem isn’t just street pharmacies or shady websites. Fake drugs can slip in through legitimate channels-diverted shipments, stolen inventory, or unauthorized resellers. A single compromised batch can spread across multiple states before anyone notices. That’s why the U.S. government didn’t just issue a warning. It built a digital backbone for every drug package.The DSCSA: The backbone of drug safety
In 2013, Congress passed the Drug Supply Chain Security Act (DSCSA). It wasn’t a quick fix. It was a 14-year plan. And it’s still unfolding. By November 2027, every prescription drug in the U.S. must be trackable from manufacturer to pharmacy-all electronically, with no paper trails. Here’s how it works:- Every box, vial, or blister pack gets a unique 2D barcode called a Unique Product Identifier (UPI).
- The barcode holds four key pieces: the National Drug Code (NDC), a serial number, lot number, and expiration date.
- Each time the package changes hands-manufacturer to wholesaler, wholesaler to hospital, hospital to pharmacy-it’s scanned and recorded.
- That data flows through a secure, standardized system called EPCIS, which handles over 15 million transactions daily with 99.95% accuracy.
Who’s responsible-and how they do it
The system doesn’t work unless everyone plays by the rules. There are four main players:- Manufacturers: They apply the UPI at the package level. For big companies like Pfizer or Merck, that’s millions of barcodes a day. They also keep digital records of every shipment.
- Wholesalers: They verify every incoming package. If something looks off, they have 24 hours to investigate. In 2023, they blocked around 12,000 suspect products before they reached pharmacies.
- Repackagers: These are companies that repackage bulk drugs into smaller doses for nursing homes or clinics. They’re a weak spot-original barcodes get destroyed. New rules now require them to reapply UPIs with full traceability.
- Pharmacies: From CVS to your local independent shop, they’re the last checkpoint. They scan every prescription before handing it to the patient. If the system flags it, they can’t dispense it.

How it compares to the rest of the world
The U.S. system isn’t the only one. The European Union uses the Falsified Medicines Directive (FMD). Here’s how they differ:| Feature | U.S. DSCSA | EU FMD |
|---|---|---|
| Serialization Format | 20-character alphanumeric code | 20-digit numeric code |
| Verification System | Decentralized, peer-to-peer data exchange | Centralized national database (EMVS) |
| Decommissioning | Optional at pharmacy | Mandatory at point of sale |
| Primary Technology | GS1 EPCIS | GS1 EPCIS + national repositories |
| Daily Verification Volume | 15+ million | 1.8 million |
The real cost-and who pays
Compliance isn’t free. For a mid-sized drugmaker, setting up serialization and EPCIS integration can cost between $500,000 and $2 million. For a small independent pharmacy? Around $18,500 a year-3.2% of their net profit. Big companies have the budget. They use platforms from TraceLink, SAP, or Movilitas. Smaller players struggle. In 2023, only 76% of U.S. pharmacies were fully compliant. Among those with fewer than 10 employees? Just 37% had the tech to handle electronic data exchange. The financial burden is real. But so is the risk. A single counterfeit drug that reaches a patient can trigger a recall, lawsuits, or worse-death. The 2022 infant formula crisis showed how fast the system can act: contaminated batches were traced and pulled from shelves in 72 hours. Before DSCSA, that would’ve taken two weeks.
What’s next? AI, blockchain, and global sync
The system isn’t static. It’s evolving.- AI is now spotting anomalies. Wholesalers are using machine learning to flag unusual shipping patterns-like a drug suddenly appearing in a region with no demand.
- Blockchain trials are underway at 34% of top pharma firms. It won’t replace EPCIS, but it adds an extra layer of tamper-proof records.
- IoT sensors track temperature and humidity in cold-chain drugs like insulin or vaccines. If a shipment overheats, the system knows-and alerts the pharmacy before it’s dispensed.
- International alignment is coming. The International Council for Harmonisation (ICH) is working on global serialization standards. By 2026, over 60 countries may use similar rules.
What you can do as a patient
You don’t need to understand EPCIS or UPIs. But you can stay safe:- Buy from licensed pharmacies only. Check the NABP’s Vetted Online Pharmacy list.
- Don’t buy drugs from websites that don’t require a prescription. The FDA estimates 96% of online pharmacies outside the U.S. are illegal.
- Check your pills. If the packaging looks off-wrong font, misspelled name, odd color-ask your pharmacist. They can scan it.
- Report anything suspicious. The FDA’s MedWatch program lets you report fake drugs anonymously.
Why this matters beyond the U.S.
The U.S. system is the largest and most advanced in the world. But counterfeit drugs are a global problem. The WHO estimates 1 in 10 medical products in low- and middle-income countries are fake. That’s over 1 million deaths a year. The DSCSA isn’t just protecting Americans. It’s setting a global standard. When a drug is made in India or Germany and shipped to the U.S., it must meet DSCSA rules. That means manufacturers worldwide are upgrading their systems-not because the U.S. told them to, but because they want to sell here. This isn’t just about compliance. It’s about trust. Trust that the medicine you take will heal you-not hurt you. And that’s worth every byte of data, every barcode, every second of verification.How do I know if my prescription drug is real?
Your pharmacist scans every prescription before giving it to you. If the barcode doesn’t match the manufacturer’s records, the system flags it and the drug is held. You can also check the packaging for a 2D barcode and ensure the lot number, expiration date, and NDC match what’s on your prescription label. If anything looks off-wrong font, color, or texture-ask your pharmacist to verify it.
Can I buy safe drugs online?
Only from pharmacies verified by the National Association of Boards of Pharmacy (NABP) through their Vetted Online Pharmacy program. Look for the VIPPS seal. Most online pharmacies outside the U.S. are illegal and sell counterfeit drugs. The FDA warns that 96% of sites selling prescription drugs without a prescription are not legitimate.
What happens if a fake drug gets through the system?
The system is designed to catch fakes before they reach patients. But if one slips through, manufacturers and distributors are required to investigate within 24 hours. They trace the package back to its source, quarantine affected lots, and notify the FDA. In 2023, over 12,000 suspect products were stopped before reaching patients. The system’s speed and precision make large-scale outbreaks extremely rare.
Why does my pharmacy ask for my ID when I pick up a prescription?
It’s not just for security. It’s part of the Authorized Trading Partner (ATP) verification process. Pharmacies must confirm they’re receiving drugs from legitimate suppliers. While ID checks don’t directly verify the drug’s authenticity, they help prevent diversion-where drugs are stolen or illegally resold. This is a layer of defense against counterfeit drugs entering the supply chain through theft.
Is the DSCSA system foolproof?
No system is perfect. Challenges remain, especially with repackaged drugs, small pharmacies lacking tech resources, and international supply chains that don’t always align with U.S. standards. Cyberattacks, like the 2023 Change Healthcare breach, can temporarily disrupt verification. But the system’s strength is its ability to detect and isolate threats quickly. Since 2015, counterfeit drug incidents have dropped by 63%, proving it works better than anything before it.





This is actually one of the most underrated success stories in public health. I used to think fake drugs were just a third-world problem, but learning how this system works made me realize how much tech and coordination goes into keeping my insulin safe. It’s not flashy, but it’s working.
Let’s be real-this whole system is just a distraction. The FDA’s numbers? Manufactured. The ‘63% decline’? They stopped counting the ones that got through. And who’s to say the barcodes aren’t cloned? I’ve seen how easy it is to spoof QR codes. This isn’t security-it’s theater. They want you to feel safe so you don’t ask questions about who really controls the supply chain.
My aunt works at a tiny pharmacy in Nebraska and she told me they almost went under trying to upgrade. The cost was insane. But now? They can scan and know for sure. I didn’t even know this existed until she told me. So glad they made it work-even if it’s messy.
So let me get this straight-you’re telling me the system that’s supposed to stop fake drugs is held together by 47 different software platforms? And we’re calling this ‘advanced’? Honey, that’s not a backbone, that’s a spaghetti junction with a barcode sticker on it.
The elegance of this system lies not in its technology, but in its humility-it does not pretend to be infallible. It acknowledges fallibility, and builds redundancy into every link. In a world obsessed with disruption, this is a quiet revolution: not of innovation, but of responsibility. Each barcode is a covenant between manufacturer and patient. A silent promise: ‘I will not let you die because I was lazy.’
Actually, you’re underestimating the complexity. EPCIS isn’t just a data format-it’s a standardized event-based messaging protocol compliant with GS1 standards, leveraging XML schemas and ISO 11783-10 for serialization. The real bottleneck isn’t tech-it’s legacy ERP integration. Most mid-tier distributors are still on AS/400 systems trying to talk to cloud-native platforms via REST APIs that weren’t designed for batch serialization. That’s why compliance lags.
It is truly admirable that the United States has implemented such a rigorous framework. In India, we are still struggling with basic cold-chain logistics. However, we must acknowledge that the DSCSA model is built upon a foundation of immense capital investment and institutional capacity-resources that are not equally available in developing nations. We must not impose Western standards without considering local realities.
They say it’s secure but nobody talks about the backdoors. Who owns the data? Who can access it? If the government can track every pill you take, what’s stopping them from tracking your health habits? This isn’t safety. It’s surveillance with a pill bottle on it
One thing people overlook: the repackagers. They’re the weak link. Imagine a nursing home getting 500 blister packs of metformin from a vendor who scraped off the original barcode and slapped on a new one. Before DSCSA, that was invisible. Now? The system flags it because the serial number doesn’t match the manufacturer’s registry. That’s huge. It’s not perfect, but it’s the first time we’ve had a digital paper trail that actually means something.
And the AI part? That’s the future. One wholesaler I spoke to uses anomaly detection to catch weird shipments-like a batch of insulin suddenly showing up in rural Wyoming with zero prescribers nearby. Machine learning flagged it. They pulled it. Turned out it was stolen. No human would’ve noticed.
The real win? Speed. In 2022, when the infant formula crisis hit, they traced contaminated batches in 72 hours. Pre-DSCSA? Two weeks. People died because of that delay. Now? We can isolate a problem before it becomes an epidemic.
Yeah, small pharmacies struggle with costs. But the alternative is worse. A single counterfeit drug can kill. And when it does, the liability isn’t just financial-it’s moral. This system doesn’t just protect patients. It protects the integrity of the entire profession.
I love how this system turns every pill into a story. It’s not just medicine-it’s a journey from factory to hand, logged like a package from Amazon. I never thought about the lot number on my blood pressure med as a digital fingerprint, but now I do. It’s weirdly comforting. Like my meds have a history, and someone cared enough to track it. Makes me feel less like a consumer and more like a person in a network of care.
Also, the fact that pharmacies are required to verify before dispensing? That’s the quiet hero moment. No applause, no headlines. Just a pharmacist scanning a box and saying ‘this is clean.’ That’s the real magic.
And the global ripple effect? When a drug made in Hyderabad has to meet U.S. standards to even enter the market, it forces change everywhere. That’s soft power at its best-not guns or sanctions, but barcodes.
my pharmacy scanned my med last week and it said ‘valid’ and i cried a little. i didn’t even know they did that. i just thought they handed me pills. turns out they’re checking if it’s poison or not. wild.
So let me get this straight-you’re telling me the government spent billions so I don’t have to worry about my pills being made of paint thinner… but I still can’t get a decent coffee in the hospital cafeteria? Priorities, people. Priorities.
And yet… the FDA still approves drugs from China that contain unapproved active ingredients. And the EPCIS system doesn’t verify the actual chemical composition-only the barcode. So what’s to stop a factory from making real-looking packages with fake pills inside? The system verifies the container, not the content. That’s not security-that’s a magic trick where you’re supposed to look at the box and forget to check what’s inside. I’ve seen the reports. The FDA’s own audits admit this gap. They’re playing whack-a-mole with counterfeiters while ignoring the real problem: they’re still letting toxic ingredients into the pipeline. This isn’t protection. It’s illusion with a barcode.
That’s a fair point. The system tracks the package, not the chemistry. But it’s still the best we’ve got. If a batch is contaminated and the barcode is legit, it’s still flagged if it’s been diverted or re-packaged without authorization. And the AI’s starting to cross-reference with lab test data from recalls. It’s not perfect-but it’s evolving. We’re not at the finish line. We’re just past the starting gate.