 17
Nov,2025
17
Nov,2025
Imagine you’re prescribed a cream for eczema. Your doctor says it’s the same as the brand-name version, just cheaper. But here’s the catch: the generic version might look identical, smell the same, and even come in the same tube - yet deliver the drug differently to your skin. That’s the hidden reality behind complex generic formulations. These aren’t your everyday pills or injections. They’re creams, inhalers, eye drops, and injectables with intricate structures that make proving they work the same as the brand name incredibly difficult - and sometimes, nearly impossible.
What Makes a Generic Drug "Complex"?
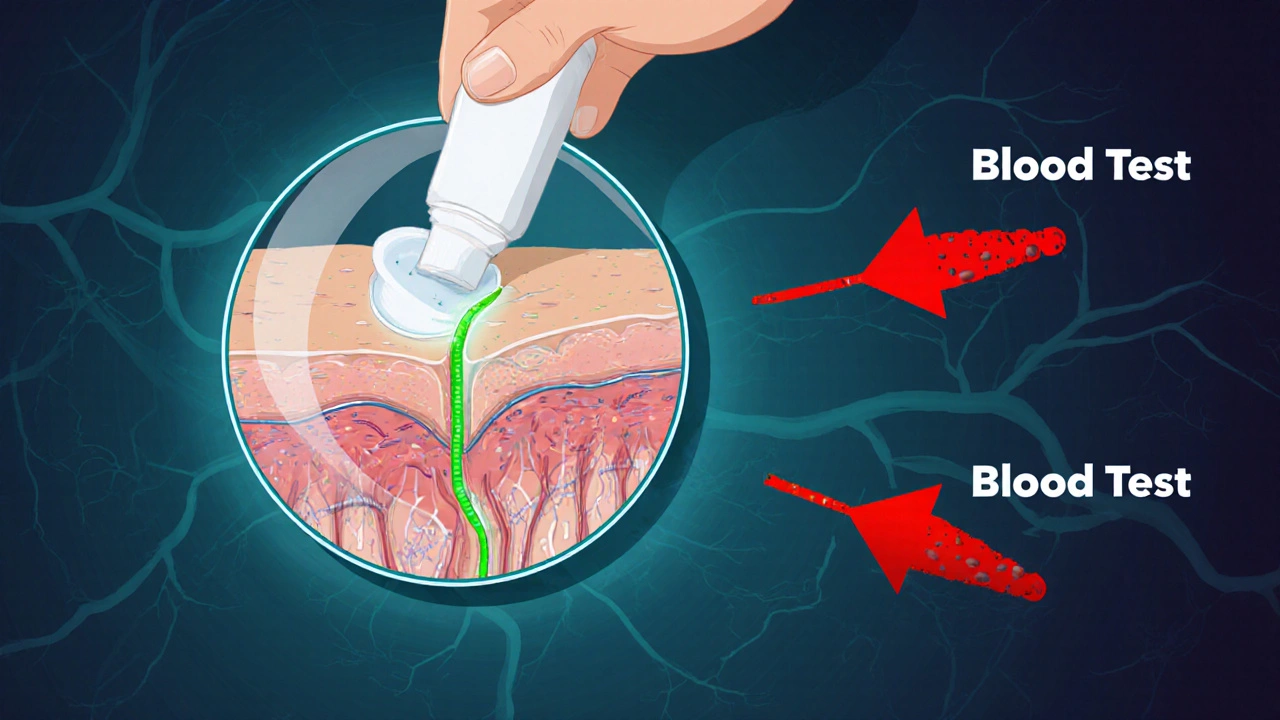
Not all generic drugs are created equal. Most are simple copies of small-molecule pills - think blood pressure meds or antibiotics - where the active ingredient dissolves in the bloodstream, and scientists can easily measure how much enters the blood. That’s straightforward bioequivalence: compare blood levels of the generic and brand drug, and if they’re within 80-125% of each other, they’re considered equivalent. But complex generics? They don’t work that way. The FDA defines them as products with hidden layers of complexity: liposomes, nanoparticles, gels, inhalers, transdermal patches, or drugs delivered directly to the skin, lungs, or eyes. These aren’t meant to flood your bloodstream. They’re designed to act locally. A steroid cream doesn’t need to show up in your blood to work - it needs to penetrate the right layer of skin. An asthma inhaler doesn’t need to be absorbed systemically - it needs to deposit the right size of particles deep in your lungs. These are the products that make up the $120 billion gap in the U.S. generic market. Over 400 brand-name complex drugs have no generic alternatives, not because no one wants to make them, but because proving they’re bioequivalent is a scientific nightmare.The Bioequivalence Problem: You Can’t Measure What You Can’t See
The standard test for bioequivalence - measuring drug concentration in the blood - fails for complex generics. Why? Because the drug isn’t meant to get into the blood. For topical products, the active ingredient stays in the skin. For inhalers, it lands in the lungs. For eye drops, it stays in the tear film. So how do you prove the generic delivers the same amount of drug to the same place? There’s no reliable way to measure drug levels in the skin or lung tissue in real time. Blood tests are useless here. That’s why regulators and manufacturers are stuck. Traditional methods can’t answer the question. And without proof of equivalent delivery, the FDA can’t approve the generic. Even when scientists try to measure local delivery, the tools are inconsistent. One lab might use a laser technique to track skin penetration; another uses tape-stripping. One inhaler manufacturer tests particle size with a laser diffraction device; another uses impaction. No global standard exists. The result? A generic approved in the U.S. might get rejected in Europe because the testing methods don’t match.The Reverse-Engineering Challenge: No Recipe, No Instructions
Generic manufacturers don’t get the original formula. They don’t know the exact ratio of ingredients, the order they’re mixed in, or the temperature and pressure used during manufacturing. All they have is the final product on the shelf. This is like trying to recreate a chef’s secret sauce by tasting it and guessing the ingredients. You might get close - but if you miss one component, the whole thing fails. For complex generics, small changes in inactive ingredients - like the type of emulsifier in a cream or the propellant in an inhaler - can drastically alter how the drug behaves. One study showed that changing the viscosity of a topical gel by just 10% reduced drug penetration by 40%. This process, called de-formulation, takes years. Companies spend millions just to reverse-engineer the reference drug. And even then, they might not know what’s critical. Is it the particle size? The crystal form? The coating? The drying process? Without the original manufacturer’s secrets, it’s guesswork.
Manufacturing Is a Minefield
Complex generics aren’t just hard to test - they’re hard to make consistently. A single batch of a liposomal injection might contain thousands of tiny vesicles, each with a slightly different size, shape, or drug load. If even 5% of those vesicles are outside the target range, the drug’s performance changes. The FDA requires manufacturers to control these variables tightly. But for products with more than 10 ingredients - common in complex formulations - the number of possible interactions explodes. Temperature changes during shipping? Humidity in the factory? A different batch of raw material? All can shift the product’s behavior. Stability testing becomes a nightmare. A cream might pass shelf-life tests in a lab, but if it’s stored in a hot bathroom for a week, the active ingredient could degrade. Or the emulsion could separate. These aren’t theoretical risks - they’ve caused real product recalls.Why Approval Rates Are So Low
While over 80% of simple generic applications get approved, only 10-15% of complex ones do. Why the drop? Because most fail at the bioequivalence stage. A 2020 survey of generic manufacturers found that 89% listed bioequivalence testing as their biggest challenge. Stability testing came in second at 76%. Characterizing the product? 68%. And these aren’t small companies - these are the biggest generic drugmakers in the world. Developing a complex generic takes 18-24 months longer than a regular one. Costs are 2.5 to 3 times higher. And failure rates at the final testing stage exceed 70%. Many companies simply walk away.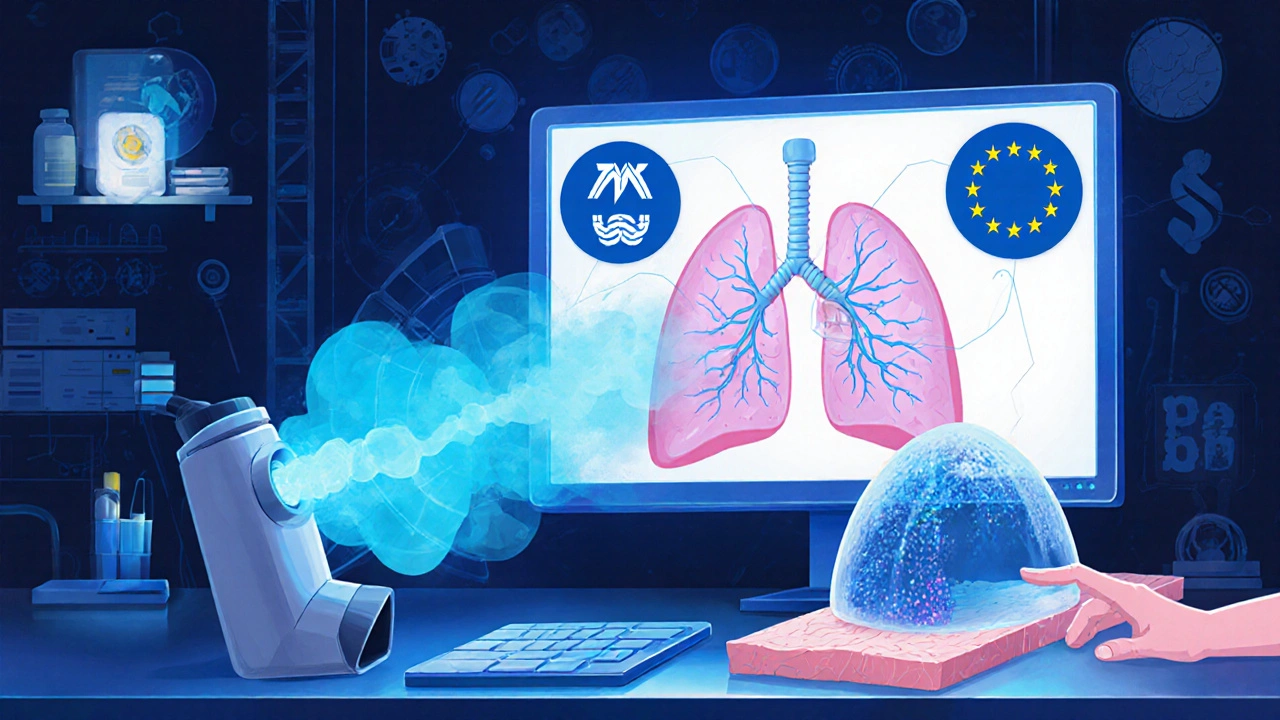
What’s Changing - And Why It Matters
The FDA knows the problem. That’s why it launched the Complex Generic Drug Product Development Program in 2022. Since then, it’s published 15 new guidance documents - for topical corticosteroids, inhaled budesonide, testosterone gels, and more. These aren’t just paperwork. They’re roadmaps. The agency is pushing for new tools: advanced imaging to track skin penetration, lung deposition models for inhalers, and computer simulations called physiologically-based pharmacokinetic (PBPK) modeling. PBPK can predict how a drug behaves in the body based on its physical properties - not just blood levels. Early results show it could cut the need for human trials by 40-60% for some products. Industry groups like the Center for Research on Complex Generics (CRCG) are working with universities to standardize testing methods. Twelve new analytical protocols were published in 2022-2023 for liposomes, nanosuspensions, and other complex forms. These aren’t just academic exercises - they’re becoming the new global benchmarks. And companies that engage early with the FDA? Their approval rates are 35% higher. That’s not luck. It’s strategy.The Future: More Access, But Not Easy
The market for complex generics is growing fast - projected to hit $45 billion by 2028. That’s a 24.6% annual growth rate. Why? Because patients and insurers are demanding cheaper alternatives to expensive specialty drugs. Think biologics for rheumatoid arthritis, inhalers for COPD, or creams for psoriasis. These aren’t niche products - they’re essential. But here’s the hard truth: making these generics will never be easy. As Dr. Steven Schwendeman puts it, "Even small manufacturing changes can have outsized effects on product performance." That’s why the future won’t be about copying - it’ll be about deep science. The next generation of complex generics will require: advanced analytics, computational modeling, standardized testing, and close regulatory collaboration. It’s no longer enough to say, "It’s the same chemical." You have to prove, scientifically, that it behaves the same in the body - even when you can’t measure it directly. For patients, this means more affordable options are coming. But they’ll arrive slowly, one hard-won approval at a time.Why This Matters to You
If you or someone you know uses an inhaler, a topical steroid, or a long-acting injection, you’re already benefiting from complex generics - even if you don’t know it. But many of these drugs are still brand-only because proving bioequivalence is too hard. The next time you hear a generic is "just as good" as the brand, ask: Is it simple? Or is it complex? For the complex ones, "just as good" isn’t just marketing - it’s the result of years of science, failed trials, and regulatory battles. This isn’t about lowering costs at the expense of safety. It’s about using smarter science to make safe, effective, and affordable medicine accessible to everyone - even when the drug doesn’t want to be measured.Why can't we just use blood tests to prove bioequivalence for complex generics?
Because complex generics are designed to act locally - in the skin, lungs, or eyes - not in the bloodstream. Measuring drug levels in the blood tells you nothing about how much drug actually reached the target site. For example, a steroid cream works by staying in the top layers of skin; if it entered the blood in large amounts, it could cause side effects. So blood tests are irrelevant and misleading for these products.
What’s the biggest hurdle in making a complex generic?
The biggest hurdle is reverse-engineering the reference drug without knowing its formula or manufacturing process. Generic companies must figure out critical quality attributes - like particle size, emulsion structure, or spray pattern - by testing the brand product repeatedly. This is like trying to rebuild a watch by only looking at its ticking, without seeing the gears inside.
Why do some complex generics get approved in the U.S. but not in Europe?
Because regulatory agencies use different testing standards. The FDA might accept an in vitro lung deposition model for an inhaler, while the EMA requires human clinical data. There’s no global agreement on how to prove bioequivalence for complex products. This forces manufacturers to run duplicate studies, increasing costs and delays.
Are complex generics less safe than brand-name drugs?
No. Once approved, complex generics are required to meet the same safety and quality standards as brand-name drugs. The FDA only approves them after rigorous testing and demonstration of equivalent performance. The challenge isn’t safety - it’s proving equivalence with limited tools.
Will complex generics ever become as common as regular generics?
Not soon - but they’re growing. While simple generics make up 90% of U.S. prescriptions, complex ones are still rare. However, with new testing methods like PBPK modeling and better regulatory guidance, approval rates are improving. Experts predict a 25-30% increase in approvals over the next five years. It won’t be fast, but it’s moving in the right direction.





This is why I stopped trusting generics. My eczema cream went from soothing to burning. They call it 'bioequivalent' but your skin knows better. 🤷♀️
THEY'RE LYING TO US. Big Pharma doesn't want generics because they can't control the narrative. They're hiding the real formulas. The FDA? Complicit. I saw a whistleblower video last week - they're using fake particle size data. You think your inhaler works? It's probably just air. 😱
While the article presents a compelling overview of the technical and regulatory challenges inherent in the development of complex generic formulations, it is imperative to recognize that the absence of standardized bioequivalence metrics does not inherently invalidate the therapeutic efficacy of approved products. The regulatory framework, though imperfect, remains grounded in empirical evidence and iterative scientific refinement.
So let me get this straight - we spend millions to reverse-engineer a cream that’s supposed to be ‘the same,’ but we can’t even tell if it’s working? And you call this healthcare? 😂 Next they’ll say a Tesla is ‘bioequivalent’ to a Model T because both have wheels.
Really appreciate this breakdown. I’ve been using a generic steroid inhaler for years and never thought about how much science goes into making sure it actually works the same way. It’s not magic - it’s meticulous, expensive, and often overlooked. Hats off to the labs doing this grunt work.
The regulatory divergence between the FDA and EMA is a significant impediment to global access. Harmonization of analytical methodologies - particularly for in vitro lung deposition and transdermal penetration assays - would substantially reduce development timelines and costs. Collaborative international standardization is not merely advisable; it is ethically imperative.
My cousin’s psoriasis cream switched to generic and it didn’t work for months until they switched back. No one told us why. Now I get it - it’s not the drug it’s the whole damn structure. The emulsifier changed and the whole thing fell apart. No wonder people are confused
Actually this is super cool science. Imagine being able to simulate how a drug moves through skin without cutting anyone open. PBPK models are the future. 🤖🧬 Also props to the FDA for actually trying to fix this instead of just ignoring it.
USA thinks it owns science but India makes 70% of the world’s generics. You think your fancy FDA tests matter? We reverse engineer your drugs in 6 months with cheaper labs. You pay 10x for nothing. This is why America is broke
It’s frustrating that progress is so slow, but the fact that we’re even talking about better testing methods means we’re moving forward. These aren’t easy problems, but the people working on them are brilliant. Keep pushing - the patients need it.
Let’s be clear - the entire bioequivalence paradigm for complex generics is a regulatory artifact designed to protect Western IP monopolies. The pharmacokinetic models are overengineered. In India we use dissolution profiling with USP apparatus IV and correlate with clinical outcomes - it’s cheaper, faster, and more predictive. The West clings to outdated methods because they can’t scale innovation.